Absolutely everything you need to know about the ATOM ... and much much MORE!
Hannah's ATOM UNIVERSE

Chemistry? more like che-MYSTERY? don't worry you have come to the right place!

Mass Spectrometer (fig 1)



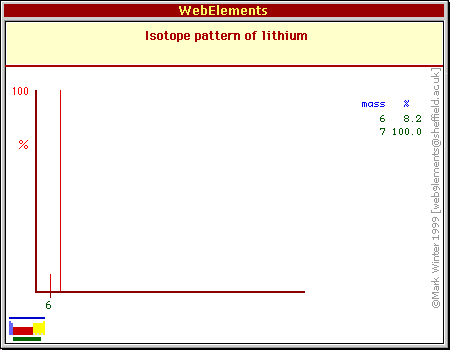
In fig 2, the two peaks are corresponding to TWO DIFFERENT isotopes of Lithium
Additional information

The mass spectrometer can also be altered so that it only focuses on/ detects positive ions with only one charge.
In this situation, the deflection is dependent on the mass alone.
Thus, the mass spectrum, is representative of the relative abundance of each isotope against the mass number, A or m/z .
Furthermore, in order to determine the relative abundance for each individual isotope, you must look at the height of each of the peaks (fig 2)

Mass Spectrometer- What is it?
The mass spectrometer refers to a device/ instrument; which is used to deduce the isotopic composition of an element.
In addition to determining the isotopic composition, it is also used to find out an element's atomic mass value. (fig 1)

THE FIVE STAGES OF THE MASS SPECTROMETER
-
VAPORIZATION
The sample is injected into the instrument where it is heated and vaporized, producing gaseous atoms or molecules.
2. IONIZATION
The gaseous atoms are bombarded by high energy electrons; generating positively charged species:
X(g) + e- -----> X+(g) + 2e-
3. ACCELERATION
The positive ions are attracted to negatively charged plates and accelerated in the electric field.
4. DEFLECTION
The positive ions are deflected by a magnetic field perpendicular to their path. The degree of deflection depends on the mass - to- charge ratio. (the m/z ratio) The species with the smallest mass, m and the highest charge, z will be deflected the most. particles with no charge are not deflected in the magnetic field.
5. DETECTION
The detector detects species of a particular m/z ratio. The ions hit the counter and an electrical signal is generated.