Absolutely everything you need to know about the ATOM ... and much much MORE!
Hannah's ATOM UNIVERSE

Chemistry? more like che-MYSTERY? don't worry you have come to the right place!

Table of comparison of subatomic particles


Prezi on Atomic Structure

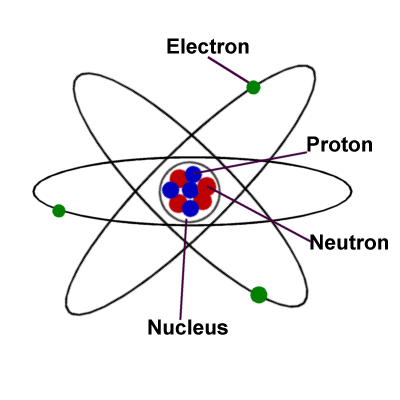
Movement of the subatomic particles in the atom
In this diagram, the blue is representing the nucleus (consisting of protons and neutrons) of the atom. The green are representative of the electrons which are constantly going around the nucleus in their orbitals.
Robert Brown, was a Scottish botanist from the 19th century. He created the concept of "Brownian Motion"
"The random movement of microscopic particles suspended in a liquid or gas, caused by collisions with molecules of the surrounding medium. Also called Brownian movement." (thefreedictionary.com)

Brownian Motion
The masses provided in the table to the left are extremely small. The amu (atomic mass unit) is a suitable unit for these masses.
1 amu = 1.660539 x 10 g
-24
Atomic Mass Unit
Nuclear Symbol
The nuclear symbol incorporates the letters A and Z. The element is represented by X
A
z
X
Element symbol
Mass Number A = Z + N
(protons + neutrons)
Atomic number= number of protons
Lithium