
Absolutely everything you need to know about the ATOM ... and much much MORE!
Hannah's ATOM UNIVERSE

Chemistry? more like che-MYSTERY? don't worry you have come to the right place!

A variety of principles exist which must be followed when depicting electron configurations
Aufbau principle
Pauli Exclusion Principle
The Aufbau principle states that electons fill the LOWEST energy orbital that is available FIRST.
The content of the Aufbau principle matches up to experimental data up to Ca Z ( atomic number) = 20. The 4s level is filled FIRST prior to the 3d level. This is due to the fact that it is LOWER in energy value.
The condensed electron configuration for Ca is written as [AR] 4s^2.
On the other hand, for the element Sc (z =21), the TWO levels can be compared in terms of their ENERGY to the 4s level. The 4s level contains a little bit more energy than the 3d level.
It is due to this, that the 3d level is filled before the 4s level.
Sc's condensed electron configuration can be read as [Ar] 3d 4s^2.
Furthermore, the Aufbau principle is supported by experimental data.
Experimental data portrays ionized 3d block elements having electrons removed from the 3d levels subsequent ot the removal of the electrons from teh 4s level.
As the 4s level is elevated in terms of energy, this theory is more reliable.
HOWEVER, the filling of thefinal three electrons is not continued throughut Sc; and thus experimental data also has not also discovered any evidence for the [Ar]3d^3 electron configuration for Sc.
3d orbitals are much more compact in comparison ot the 3s orbitals; Thus the electrons which enter the 3d orbitals tend to go through a higher rate of MUTUAL REPULSION.
Nuclear change increases as we move through the atoms.
The Pauli exclusion principle states that any orbital can hold a maximum of two electrons. And these electrons have opposite sign.

Hund's rule of maximum multiplicity states that when filling degenerate orbitals (orbitals of equal energy), electrons fill all the orbitals singly before occupying them in parts.

Hund's law of maximum multiplicity

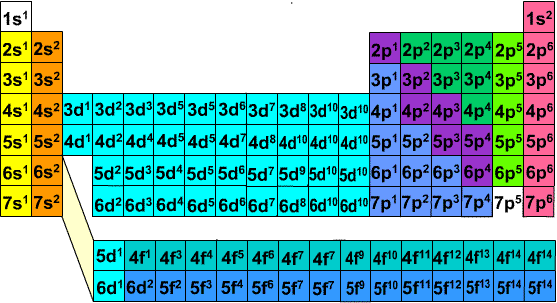
FIGURE 1: ORDER OF ENERGY LEVELS OF FIRST FEW SUBLEVELS
Electron congigurations can be portrayed in three ways
1) full electron configuration
2) Condensed electron configuration
3) Orbital diagram representation
* when wiriting electron configurations, tHe periodic table is used to help "build up" the electrons in order of their orbitals; in respect to the three methods provided above
Electron configurations

FOR MORE INFORMATION ON SUBLEVELS CLICK HERE
Table of first 36 elements with Full electron configurations
The blocks of the periodic table correspond to the sublevels s, p, d, and f
Cu and Cr have different electron configurations than what would normally be expected.
Cu (Z= 24)
Cu (Z = 29)
Cr = 1s2 2s2 2p6 3s2 3p6 3d5 4s1
Cu = 1s2 2s2 2p6 3s2 3p6 3d10 4s1
Electrons enter the 3d orbitals PRIOR to filling (completely) the 4s orbital.
Chromium has a half filled 3d sublevel which also consists of 5 electrons, whilst copper has a completely filled 3d sublevel of 10 electrons.
3d sublevels which are half filled and completely filled also reduce an atom's overall potential energy.
The electron configurations 3d 5 4s1 and 3d10 4s1 are more stable than 3d4 4s2 and 3d9 4s2.
Exception: Chromium and Copper
An element's chemistry is based on its outer VALENCE ELECTRONS (in contrast to the inner CORE ELECTRONS)
The CONDENSED ELECTRON CONFIGURATION IS A MUCH MORE suitable method of representing electron configurations :
[nearest noble gas core] + valence electrons
For example;
He = [He]
O = [He] 2s2 2p4
Ne = [He] 2s2 2p6 or can be written as [Ne]
P = [Ne] 3s2 3p3
Condensed electron configuration
Click above to expand your knowledge further and learn about orbitals, and other fascinating concepts!
